Abstract
Background: Bruton tyrosine kinase inhibitors (BTKi) and fixed-duration obinutuzumab/venetoclax (OBIN/VEN) have evolved as the standard-of-care frontline treatments for patients with chronic lymphocytic leukemia (CLL). However, few studies have compared their efficacy and safety. The phase 3 randomized, non-inferiority trial, CLL17, which compares ibrutinib with OBIN/VEN and VEN/ibrutinib, is currently recruiting. However, real-world evidence is lacking.
Method: Data were collected from the TriNetX Research Network, an aggregated database linking tumor registries, claims, and electronic health records from 66 healthcare organizations. Patients with CLL were identified through ICD-10-CM codes. From 6/2016 to 6/2022, patients with CLL receiving OBIN/VEN as the frontline treatment were matched 1:1 based on propensity score to those receiving BTKi monotherapy. Patients with TP53 alterations were excluded.
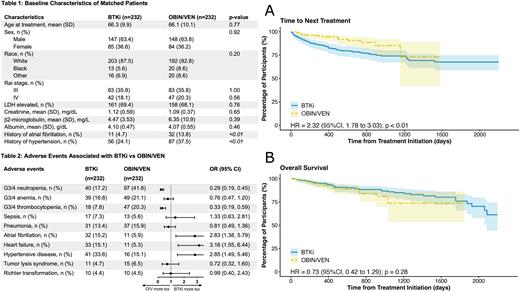
Results: During the study period, a total of 232 and 2,560 adult patients received OBIN/VEN and BTKi monotherapy as the frontline treatment, respectively. After propensity score matching, 232 patients were identified in each group. They were well balanced regarding their baseline characteristics, including age, sex, race, Rai stage, LDH elevation, creatinine, b2-microglobulin, and albumin level (Table 1). After a median follow up of 457 and 882 days, 18 and 56 patients in the OBIN/VEN and BTKi group received next line of therapy, respectively. Time to next treatment (TTNT) was significantly shorter in the BTKi group compared with the OBIN/VEN group (hazard ratio [HR] 2.32, 95% confidence interval [CI] 1.78 to 3.03; p<0.01; Fig A). Of the 18 patients in the OBIN/VEN group who received next-line treatments, 3 received BTKi while the rest received chemoimmunotherapy; the median TTNT was 296 (range 48 to 1,157) days. Altogether, 24 and 37 deaths occurred in the OBIN/VEN group and BTKi group, respectively; there was no significant difference regarding the overall survival (OS) (HR 0.73, 95%CI 0.42 to 1.29; p=0.28; Fig B). Lastly, common adverse events were investigated between the two groups (Table 2). Patients receiving OBIN/VEN were associated with significantly higher incidences of grade 3 or 4 neutropenia and thrombocytopenia. However, there was no significant difference regarding infectious outcomes such as sepsis or pneumonia. There was also no significant difference of tumor lysis syndrome. Expectedly, patients receiving BTKi were associated with significantly higher incidences of atrial fibrillation, heart failure, and hypertension, when compared with OBIN/VEN (Table 2).
Conclusions: In patients with CLL and without TP53 alteration who received frontline treatment, OBIN/VEN was associated with significantly longer time to next-line treatments than BTKi. The difference of OS was not significant. OBIN/VEN was associated with more cytopenia, while BTKi was associated with more cardiac toxicities.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal